Consortium
ACADEMIC RESEARCH ORGANIZATIONS
UNIVERSITIES
EUROPEAN COUNTRIES INVOLVED
HT-ADVANCE builds on the complementary and internationally recognised expertise of the partners in the fields of HT, clinical endocrinology, omics technologies, biomarker development and validation, big data handling and modelling, clinical methodology, health economics, ethics research and innovation, providing a unique interdisciplinary consortium addressing all major challenges related to the program:
- Clinical excellence centres for arterial HT and adrenal diseases (ESH Excellence centres and ENS@T reference centres): APHP, RADBOUD, UNITO, UNIPD, CUS, UZH
- Clinical and research laboratories, experts in omics measurements: Inserm, TUD, HMGU, UGLA, APHP, UoB
- Health informatics and bioinformatics, and experts on methodology: UNIVDUN, Inserm
- Health economics: APHP
- Ethics: UT3
- Project management and technology transfer: IT
A complementary consortium
The HT-ADVANCE consortium has emerged from a previous partnership developed within the H2020 ENSAT-HT program (GA No 633983, coordinated by MC Zennaro) that has allowed it to generate an interactive network between members and across various disciplines. The experience of the interdisciplinary approach has been expanded in HT-Advance to address ethics, legal and regulatory issues related to the project developments and innovative products based on Artificial Intelligence, MOMICS and personalized medicine approaches for the benefit of patients. Combined expertise on economical, ethical and regulatory issues, implementation and IP management will allow producing a management plan for implementation of MOMICS biomarkers as companion diagnostics.
A consortium of excellence
The HT-ADVANCE consortium builds on complementary and internationally recognized multidisciplinary expertise and proven track record of the partners in the fields of arterial HT, clinical endocrinology, omics technologies, biomarker development and validation, big data handling and modeling, clinical methodology, health economics, ethics research and innovation management of health products. Clinical centers participating in the consortium are European Society of Hypertension Centres of Excellence and members of the European Network of adrenal tumors ENS@T, providing a unique capability for the recruitment and workup of patients with primary and secondary hypertension.
Other countries and international organizations
The HT-ADVANCE consortium includes three universities from the UK (UNIVDUN, UGLA and UoB) which are associated partners in HT-Advance. The project also includes a Swiss University (UZH) as an associated partner.
Industrial/commercial involvement
The MOMICS biomarkers will be validated for clinical use within HT-Advance. WP4 will establish a development strategy to prepare translation to clinical use of the biomarker including the formulation of a product design strategy, the regulatory approach and finally the business plan, which will pave the way for the exploitation not only of MOMICS-ENDO, but also of MOMICS-TREAT biomarker.
Consortium Map
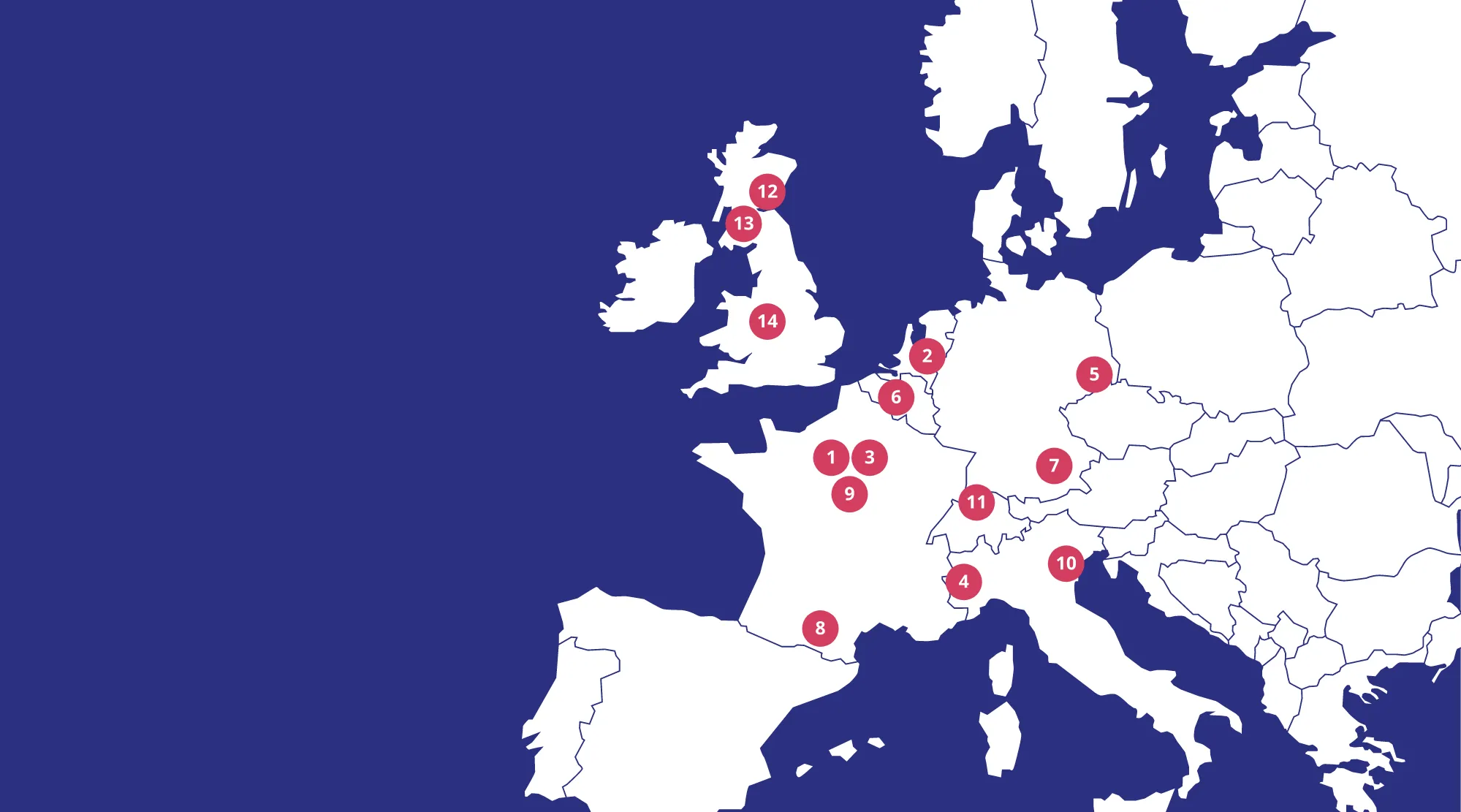
INSTITUT NATIONAL DE LA SANTÉ ET DE LA RECHERCHE MÉDICALE
(INSERM) FRANCE
STICHTING RADBOUD UNIVERSITAIR MEDISCH CENTRUM
(RADBOUDUMC) NETHERLANDS
INSERM TRANSFERT SA
(IT) FRANCE
UNIVERSITÀ DEGLI STUDI DI TORINO
(UNITO) ITALY
TECHNISCHE UNIVERSITÄT DRESDEN
TUD) GERMANY
CLINIQUE UNIVERSITAIRE SAINT-LUC ASBL
(CUS) BELGIUM
HELMHOLTZ ZENTRUM MÜNCHEN DEUTSCHES FORSCHUNGSZENTRUM FÜR GESUNDHEIT UND UMWELT GMBH
(HMGU) GERMANY
UNIVERSITE PAUL SABATIER TOULOUSE III
(UT) FRANCE
ASSISTANCE PUBLIQUE HÔPITAUX DE PARIS
(APHP) FRANCE
UNIVERSITÀ DEGLI STUDI DI PADOVA
(UNIPD) ITALY
UNIVERSITAT ZURICH
(UZH) SWITZERLAND
UNIVERSITY OF DUNDEE
(UNIVDUN) UNITED KINGDOM
UNIVERSITY OF GLASGOW
(UGLA) UNITED KINGDOM
THE UNIVERSITY OF BIRMINGHAM
(UoB) UNITED KINGDOM






